Acute myeloid leukaemia is a cancer of the blood cells. The blood cells are cells in the bone marrow and blood stream that protect people against infections and provide immunity to the body with white blood cells, transport oxygen from lungs to all parts of the body with red blood cells and help with clotting of blood with platelets. All these blood cells start with a stem cell in the bone marrow. The stem cell divides into a myeloid stem cell and lymphoid stem cell. The myeloid stem cell then divides into myeloid blast cell which in turn divides into neutrophils and monocytes. The myeloid stem cell also gives rise to red blood cells and platelets. Acute myeloid leukaemia is a cancer of the myeloid stem cell. In AML, there is excessive production of blast cells, immature neutrophils and monocytes.
While the cancer increases and occupies most of the space in the bone marrow, it prevents the production of other blood cells in the body such as other white blood cells, platelets, red blood cells etc. This leads to the symptoms produced by the cancer. The cancer can spread to other parts of the body such as lymph nodes, spleen or nervous system.
Unlike chronic myeloid leukaemia which tends to develop slowly, AML grows in a patient quickly over days or weeks.
Acute myeloid leukaemia can present in children and adults but is more common in adults, particularly in the older age group with 40% of AML occurring in patients over the age of 75.
Acute myeloid leukaemia is divided into different types based on the different classifications that are used. The two main classifications used are the FAB classification and WHO classification.
FAB (French- American-British) Classification
FAB classification divides the AML into 7 subtypes from M1 to M7. These are listed below.
M1-AML without maturation
M2- AML with maturation
M3- Acute promyelocytic leukaemia
M4- Acute myelomonocytic leukaemia
M5- Acute monocytic leukaemia
M6- Acute Erythroleukaemia
M7- Acute megakaryoblastic leukaemia
WHO Classification
The WHO classification is based on the type of leukaemia cell that is found, the genetic changes that are present in the leukaemia, AML that has occurred due to previous chemotherapy and the presence of another blood condition that may have caused the acute leukaemia. The detailed list is not given here.
The risk factors associated in the development of acute myeloid leukaemia are listed below. Having these risk factors increases the risk of getting AML.
Age
Older age is a risk factor for AML. About 40% of AML occurs in patients over the age of 75.
Genetic conditions
People associated with the following genetic conditions have an increased risk of developing leukaemia.
- Down’s syndrome
- Li-Fraumeni syndrome
- Bloom syndrome
- Ataxia Telangiectasia
- Neurofibromatosis type 1
- Wiscott-Aldrich syndrome
Radiation
People exposed to high levels of radiation in the past have a risk of developing acute leukaemia. The risk is higher for acute myeloid leukaemia than acute lymphoblastic leukaemia. Previous radiotherapy for other cancer or condition carries a risk for developing a leukaemia.
Previous chemotherapy
Children who had previous chemotherapy are at increased risk of leukaemia some years after treatment. This risk is more for acute myeloid leukaemia than ALL.
Smoking
Smoking is a significant risk factor for the development of AML. Benzene is present in tobacco which increases this risk.
Exposure to Benzene
People working in petroleum and chemical industry who are exposed to benzene which they inhale is a risk factor for the development of Acute myeloid leukaemia.
Blood disorders or conditions
Disorders of the blood such as polycythemia vera, myelodysplastic syndrome, myelofibrosis and chronic myeloid leukaemia can all predispose to the development of AML.
Overweight and Obesity
Being overweight or obese slightly increases the risk of developing AML.
AML can produce a variety of symptoms due to its effect on the blood, bone marrow, lymph nodes, liver, spleen and other parts of the body. Common symptoms associated with it are given below. It is important to note that these symptoms can happen due to other reasons and most patients who have these symptoms do not have a leukaemia.
Weakness and Tiredness
This is a common symptom that is present in patients with this condition. It can be due to anaemia as enough red blood cells are not being produced due to this condition. The patient notices tiredness on performing small tasks which they were able to do before.
Breathlessness
Breathlessness is also a symptom of anaemia as there are lesser number of red blood cells to transport oxygen to the tissues. This leads to the patient needing to put more effort in breathing to do the same amount of work.
Fever
Fever is a common symptom of AML.
Repeated Infections
Patients with AML can have repeated infections due to reduced immunity. This is because of reduced production of normal white blood cells and reduced function of the white blood cells that are produced. The capacity for the body to fight infections will be less and hence patients can experience more infections.
Bleeding and bruising
There can be reduced number of platelets in patients with AML because of reduced production of them in the bone marrow and increased destruction of platelets in the body. As platelet function is to help with blood clotting, a reduction of these cells can lead to increased episodes of bleeding. The bleeding can be from anywhere in the body including the gums, nose, while passing motion or urine etc. Bruising may be visible on the skin.
Lymph node enlargement
Lymph nodes are present in all parts of the body including the neck, under the arms, chest, abdomen, pelvis and groins. Enlargement of these lymph nodes can be a symptom of AML and this leads to swelling in these areas. Enlargement of nodes in the chest can lead to cough and breathlessness.
Swelling and fullness in the Abdomen
Some patients with AML have enlargement of organs such as liver and spleen. Both organs are present in the abdomen, and this enlargement leads to distension or a feeling of fullness in the abdomen, aching sensation or breathlessness.
When AML is suspected, the following tests are done to aid in diagnosis and to decide on treatment options. These are done after a thorough examination by the doctor.
Blood tests
Routine blood tests such as a full blood count or complete blood picture will inform about the number of red blood cells, white blood cells and platelets in the blood. It could also indicate the presence of leukaemia cells if present in the blood. Other blood tests include kidney function and liver function tests, bleeding and clotting profile of the blood, Lactate dehydrogenase (LDH) and uric acid.
Flow Cytometry
This is a process where blood cells are further characterised based on their physical and chemical properties. A flow cytometer is a machine which analyses the blood sample using a laser. It uses antibodies and fluorescence to locate the cells. The cells are named or separated by the different fluorescence produced by them. This is picked up and analysed by a computer. It helps in making a diagnosis of Acute myeloid leukaemia and subtype the different types of AML. Flow cytometry is a more sensitive test than standard microscopy and is used to diagnose and to detect recurrence of AML after initial treatment.
Bone Marrow Examination
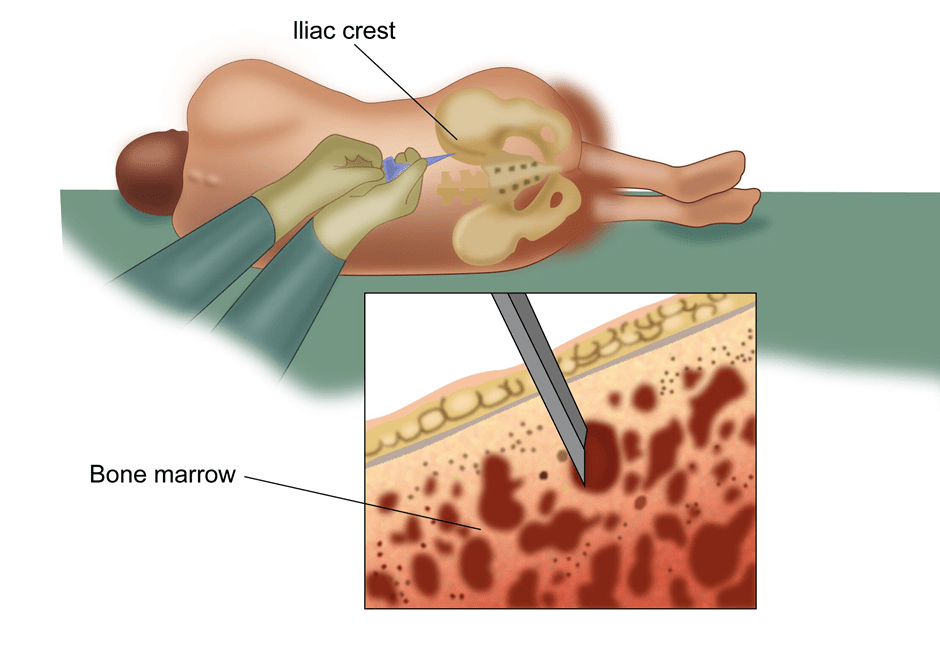
A bone marrow examination involves taking a sample of the marrow from the bone to look for cancer cells (AML) and other abnormalities in the bone marrow. This test is commonly done in cancers related to the blood and lymphatic system. The marrow is taken from the bones of the pelvis or sternum. A local anaesthetic drug is given to reduce the discomfort prior to the procedure. The test is done on an outpatient basis and the patient can go home after the test is done. Two types of examination are done. One is bone marrow aspiration where a sample of fluid from the marrow is taken and the other is a trephine biopsy where a small piece of the bone is also taken. The report will be available in a few days.
Cytogenetics
Samples from the bone marrow biopsy are also tested for changes in the genetic material in the cells which can help in diagnosis of the subtype of AML and inform about the possible treatment options and outcomes of the condition after treatment.
Immunophenotyping and Immunohistochemistry
These tests look at the proteins on the cells using antigen and antibody reactions to exactly find and type the cells of AML. The antibodies used to detect the antigens on the cells are fluorescent which enables the examiner to see the cells.
FISH and PCR
These are tests used to diagnose leukaemia by looking at the genetic changes by using DNA probes
Lumbar Puncture
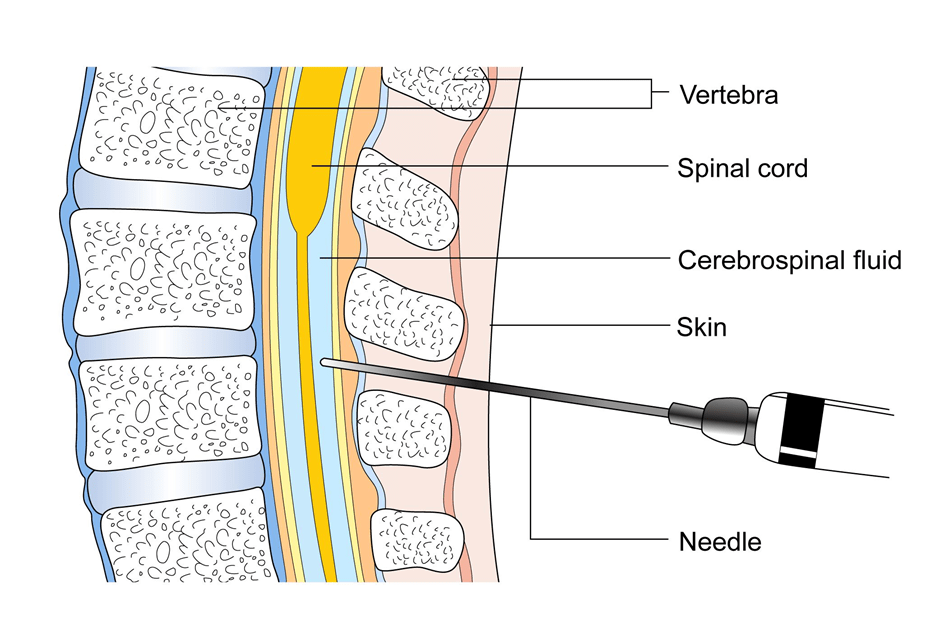
This is a test where is a needle is placed in the spine to take a sample of fluid present in the area surrounding the spinal cord to look for the presence of leukaemia cells. This test is done under local or general anaesthetic depending on the age of the patient.
CT Scan
A CT scan is done in patients with AML to look for the presence of enlarged lymph nodes or organs such as liver and spleen. The scan will aim to cover the areas of the neck, chest, abdomen and the pelvis.
MRI Scan
An MRI scan is considered if there is suspicion of the cancer spreading into the brain. In this setting, an MRI is better than a CT scan.
US scan
An ultrasound scan of the abdomen is done to look for enlargement of the liver or spleen if a CT scan is not being considered.
Treatment of Acute myeloid leukaemia depends on the age of the cancer at diagnosis, the fitness of the patient, the extent of the disease and the subtype of AML. Generally, the treatment can be divided into phases which are induction phase, consolidation and maintenance phase.
The main type of treatment used in these three phases is chemotherapy, which is use of drugs given through a vein. Chemotherapy is also given into the spine to prevent relapse or to treat disease that is in the nervous system. Other forms of treatments used include targeted or biological therapy, radiotherapy and stem cell transplant. These are discussed below.
Chemotherapy
Induction Phase
The induction phase of chemotherapy also known as remission induction phase involves the use of a combination of drugs with the aim to achieve a complete remission, wherein there are no remaining AML cells in the blood or bone marrow. Treatment is given on an in-patient basis and patient stays in hospital for some time, usually weeks, to be monitored for blood counts and side effects of treatment. Drugs that are commonly used as chemotherapy for induction phase include
Cytarabine along with Mitoxantrone or Daunorubicin or Idarubicin. These are called as “7+3” treatments where Cytarabine is given daily for 7 days and is followed by one of the other drugs for 3 days.
Certain types of AML such as those with FLT3, IDH1 and IDH2 mutations may also benefit from targeted drugs such as Midostaurin, Ivosidenib or Enasidenib respectively.
Treatment is usually quite intensive and potential side effects include tiredness, nausea, vomiting, constipation, hair loss, loss of taste and appetite, loose motions, soreness in the mouth, low blood counts and risk of infection.
In patients who have low blood counts, injections to help improve the white cell count (growth factors), blood or platelet transfusions may be needed. Antibiotics are given for patients with infection after treatment. The low blood counts recover slowly over time.
For patients who are less fit, a less intensive induction treatment is planned with drugs such as Azacitadine, Decitabine, low dose Cytarabine and Gemtuzumab ozogamicin. The Oncologist or Haematologist decides on the best approach based on the patient’s condition.
A bone marrow aspirate and biopsy is done at 7 days and again in 14 days if required to look for assessment of response to chemotherapy.
Consolidation Phase
Consolidation therapy, which is again intensive chemotherapy is given to increase the chances of a cure in patients who have complete remission after induction chemotherapy. Without consolidation chemotherapy, the risk of recurrence of the AML will be very high. This phase of treatment includes courses of chemotherapy with high dose Cytarabine (HiDAC), Daunorubicin, Idarubicin, Mitoxantrone etc or autologous/allogenic stem cell transplant. If targeted therapy was used at induction treatment, the same can be continued in this phase too. The selection of treatments such as chemotherapy only or chemotherapy followed by stem cell transplant or directly to stem cell transplant depends on many factors such as the type of AML, the genetic changes in AML, the fitness of the patient and the risk profile of the disease based on the above features. Patients can be classified into favourable profile, intermediate risk profile or poor risk profile and decisions regarding the most appropriate consolidation treatment will take these factors into account.
Stem Cell Transplant
Stem cell transplant is a type of treatment that may be used in AML in patients who had a complete remission and have an intermediate or high risk profile of disease and in patients who are less than 60 years of age. Those patients who have a normal risk of relapse may not need a stem cell transplant.
Prior to having a transplant, the patient is given a high dose of chemotherapy called conditioning chemotherapy. The aim of this chemotherapy is to kill off all AML cells (myeloablative conditioning). Non myeloablative conditioning is given to older or less fit patients, where the chemotherapy is less intensive. A combination of chemotherapy and radiotherapy can also be used as a conditioning treatment prior to a transplant.
The function of the bone marrow normally is to produce blood cells such as red blood cells which help the blood to carry oxygen, white blood cells which protect against infections and platelets which help stop bleeding. A significant lowering of these cells in the blood is dangerous to the patient and hence a transplant of these cells is needed after a high dose of chemotherapy.
Collection of Stem Cells
Stem cells are a type of blood cells that have the capacity to develop into any kind of blood cell such as red blood, white blood cell or platelets. These stem cells are present in the blood stream and bone marrow and are initially collected from the patient before the patient receives high dose chemotherapy. This process of collection of stem cells from the patient and infusing them back into the same patient after high dose chemotherapy is called as Autologous Stem cell transplant.
If the stem cells are from another person(donor), then it is called an Allogenic stem cell transplant. The donor can be related, usually a brother or a sister, or unrelated but matched or partially matched donor. An allogenic transplant is preferred in AML if a donor is available.
Before collection of stem cells, the patient/donor is given injections with G-CSF which will increase the number of stem cells in the blood to achieve a successful collection.
On the day of collection of stem cells, the patient (auto) or donor (allogenic)is connected to a machine and the blood is taken out from one vein and it passes through the machine to collect the stem cells present in the blood. The blood then passes back into the patient/donor through another vein. This process is done over a few hours.
Once the stem cells are collected, the patient receives the high dose chemotherapy. After the chemotherapy, the stem cells are infused into the patient. These cells go into the bone marrow and start making blood cells again.
Collection of bone marrow
Bone marrow is the spongy material that is present inside the bones. For a bone marrow transplant, the marrow needs to be collected prior to giving high dose chemotherapy. The procedure to collect the marrow is done under general anaesthesia usually in an operation theatre. The marrow may be taken out from different places in the bones and about 1 litre of it may be taken out at the procedure. Once taken out, it is stored and infused into the patient when needed.
Risks and side effects of Stem cell Transplant
Having a stem cell or bone marrow transplant is a complex process and is associated with side effects. This procedure usually involves staying in hospital for a few weeks for the blood cells in the marrow and blood to recover to normal levels after the transplant is done. Common side effects associated with this procedure include
Nausea, Vomiting, Hair loss, altered function of the liver are potential side effects of this treatment.
Risk of Infection as white blood cells are low and the patient to prone to get an infection. The infections could be bacterial, viral or fungal and will usually need antibiotics to control them.
Mucositis is the effect of chemotherapy on the inside lining of the mouth and digestive tract. This can limit the amount of food taken by the patient and other methods of feeding may be used in that instance.
Bleeding is a risk associated with this procedure due to low platelet count, but platelet transfusion can be given to keep the platelet counts at required levels.
Graft versus host disease is a reaction of the body to the transfused cells particularly if the stem cells or marrow is from a donor.
Radiotherapy
Radiotherapy may also be used as a conditioning treatment prior to a stem cell transplant. Conditioning treatment is one where the treatment is aimed to get rid of all blood cells in the body prior to the transplant of stem cells. This conditioning treatment can be chemotherapy or radiotherapy. When radiotherapy is used, it is given to the whole body and is called as total body irradiation (TBI). Side effects of TBI treatment include tiredness, sleepiness, hair loss, loose motions, soreness in the mouth, low blood counts and risk of infection, bleeding, nausea and vomiting
Treatment of relapsed or residual disease
A good proportion of patients in induction and consolidation treatment will go into remission. Some patients will not and will have to be treated with other drugs. A proportion of patients who have had a remission after above may develop a relapse of disease sometime later. Treatment options for these patients may include more chemotherapy followed by stem cell transplant if they have achieved a partial or complete remission after treatment again.
Graft versus host disease is a complication of stem cell or a bone marrow transplantation. It is a reaction between the donor’s cells (T cells) that have been transfused into the patient and the patient’s body. The T cells see the host’s (patient) cells as foreign and react to them. The skin, intestine and liver are the organs mostly affected by this condition. GvHD happens in patients with allogenic transplant, where cells that are transplanted from another person. The risk of this complication is lesser if the transplant donor is closely related and there is a good match between the donor and patient. The risk is more if an unrelated donor or a partially matched donor is used.
GvHD can be of two types, acute and chronic. GvHD can also be graded according to its severity.
Grade 1- patient has mild symptoms
Grade 2- moderate symptoms
Grade 3- severe symptoms
Grade 4- very severe symptoms
Acute GvHD is when the complication happens in the first 100 days of the transplant, but it usually happens 2-3 weeks after the transplant. Symptoms of acute GvHD include skin rashes which can be itchy and painful. Other symptoms include loose motions, lack of appetite, vomiting and jaundice (yellowing of eyes).
Chronic GvHD occurs 100 days after the transplant and symptoms of that include skin rashes which can be itchy, skin discolouration, loose motions, dry mouth, jaundice and scarring in the liver. It can affect the lungs leading to cough and breathlessness, eyes causing dryness and pain, aches and pains in the muscles.
A diagnosis of GvHD is made based on the symptoms of the patient and the findings on examination by the doctor. A biopsy of the skin, liver or other area is taken to confirm its presence.
Treatment and Prevention of GvHD is mainly achieved by suppressing the immune system to reduce the reaction between the donor and host cells. Drugs are used to help with this. Commonly used drugs include Infliximab, Etanercept, Mycophenolate mofetil (MMF), Sirolimus, Tacrolimus, Rituximab, Ibrutinib, Azathioprine and Pentostatin. Common supportive treatments such as pain killers, medications to reduce vomiting, loose motions, antibiotics to reduce or treat infections, artificial tears to prevent dry eyes, nutritional and fluid support and other measures are used to help control the condition. Patients who have chronic GvHD can have symptoms that are present over a long period of time and supportive measures for the skin, liver, lungs, eyes, vagina are continued. Necessary precautions




